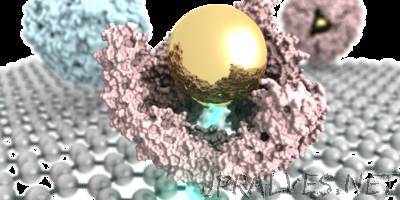
“Physicists at the University of Pennsylvania have invented a new type of graphene-based sensor that could one day be used as a low-cost diagnostic system able to test for biomarker molecules, which are indicative of disease states. In collaboration with Penn chemists, the researchers published a paper in Chemical Science in which they use this sensor, for the first time, to directly answer a basic scientific question in nanotechnology about whether a particular protein maintains its structure when it assembles around an inorganic nanoparticle. The finding may have medical applications down the line. The research was led by Jinglei Ping, a postdoctoral researcher in the Department of Physics & Astronomy in Penn’s School of Arts & Sciences, physics professor A.T. Charlie Johnson and Katherine Pulsipher, previously a grad student in the Department of Chemistry and now a postdoc in the Department of Chemical and Biomolecular Engineering in the School of Engineering and Applied Science. Chemistry professors Ivan Dmochowski and Jeffery Saven, graduate students Ramya Vishnubhotla and Jose Villegas and alumnae Tacey Hicks and Stephanie Honig also contributed to the study. This unique sensor, which the researchers call a graphene micro electrode, works by measuring the current that flows from a liquid sample to the graphene surface. The researchers found that this technique is especially useful in more complicated liquid samples, such as biological fluids, which typically have a lot of salt and proteins. Dmochowski’s group has been studying the interaction of inorganic nanoparticles with proteins for the past decade. Specifically, the researchers have been looking at a type of protein called ferritin, which is better known for being an iron-storage protein. A particular thermophilic ferritin has the unusual feature of assembling in high salt and disassembling in low salt. “What we found some years ago,” Dmochowski said, “is that we could take salt out of the equation and work in low salt where the protein would normally be disassembled and put in a gold nanoparticle with a coating that makes it very charged in solution with the ferritin. The nanoparticle is spontaneously encapsulated by the protein, like a ship in a bottle. The protein cloaks the nanoparticle and changes its properties in solution.” Inorganic nanoparticles are widely considered potential next-generation diagnostic and therapeutic agents. However, they are known to interact with and denature proteins, which form a thin shell around the nanoparticle called a corona. “That’s not what happens with our protein,” Dmochowski said. “It has 24 different subunits that come together to encapsulate the nanoparticle, and we have found over the years that it maintains much of its native structure.” But the researchers had an unresolved question. One characteristic feature of this protein is that is has four triangular nano-sized pores. The researchers wanted to know whether these pores are maintained when the protein assembles around the gold nanoparticle. Although there are several techniques that might be able to resolve this challenging imaging question, Johnson said, they tend to be a bit more indirect and cumbersome.”
