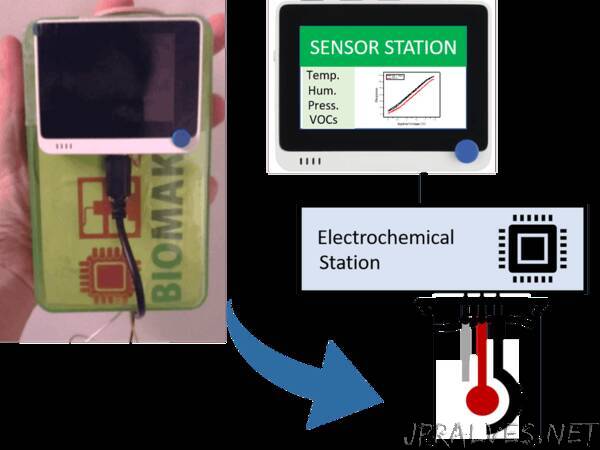
“We report a handheld, portable potentiostat with a user-friendly interface for electrochemical sensing using a wireless power supply.
1. Synopsis
The development of reliable and low-cost electrochemical potentiostats is essential in the field of chemical sensors and biosensors. These potentiostats can be used for the calibration of sensors, and the use of such sensors for the testing of samples. Although there is a wide range of commercially available potentiostats that can be used for analysis and research nowadays, these options tend to be expensive, restricting their use. Within recent years, several low-cost potentiostats have been reported, employing simple designs with Op-amp amplifiers. However, the operation of these low-cost potentiostats tend to be complex, requiring programming skills to tailor the needed parameters according to the experimental conditions.
This project aims to incorporate the design of a low-cost potentiostat with an intuitive interface to allow the testing of electrochemical sensors. We employed a Wio-Terminal which includes a lcd screen and configurable buttons, and has a range of sensors incorporated that could be further developed to improve the capabilities of this electrochemical potentiostats. This system is based on the standard three-electrode cell configuration and incorporates a sensor for the evaluation of environmental conditions. We employed this potentiostat for the testing of an electrolyte solution using a screen-printed electrode, and the monitoring of plant stems, although we envision that the applications of this project can be extended to multiple other systems and sensors.
2. Sensor Overview
The figure below illustrates the electrical design of the potentiostat. This system required an analog output, that allowed the application of a specific voltage, and the gradual increase of its magnitude. Such analog output was provided by a MCP4725, a readily-available digital-to-analog converter (DAC) that can communicate to a microcontroller via I2C. This DAC was then connected to an Op-amp, where the reference and counter electrodes were located. For the testing of this potentiostat, we employed a screen-printed carbon electrode. A working electrode was additionally added, which was connected to a current to voltage converter. This current to voltage converter allowed us to measure the response of the sensors by transforming the current response of the sample into a voltage, that can be measured by our microcontroller.
This system was enclosed in a plastic box for its portability, although this project could potentially be further miniaturised by employing a printed circuit board. We additionally included a BME680 environmental sensor inside the box to allow the determination of some crucial environmental parameters such as the temperature, humidity and pressure. All the values obtained from this sensor along with the data extracted from the potentiostat were displayed on the lcd screen of a Wio Terminal. This device could be used for the design of an intuitive interface that could not only display the obtained data, but also the experiment parameters could be directly tuned by the user through the push buttons on the device.
3. Description of the device
The potentiostat was adapted for conducting Stripping Voltammetry experiments, which can be used for the determination of ions. In this electrochemical technique, an increasing voltage is applied within the counter and working electrodes, and the resulting current is measured. As such, three main parameters can be modified for this experiment; The initial voltage, final voltage, and speed of change. To allow the user to change these parameters, we made use of the back push buttons of the Wio Terminal, and designed an interface to change these parameters and plot the data on the lcd screen as can be seen in the figure below.
Initially, the potentiostat was tested on a sodium chloride solution using Cyclic Voltammetry. This test was repeated on water without sodium chloride for comparison. The results from the testing of the potentiostat being employed for Cyclic Voltammetry are shown on the figure above. As a consequence of the increase in the concentration of electrolytes in the water dispersion, the potential response of the sensor increased as expected given the drop in the resistance of the solution due to the increase of NaCl ions. In addition, an hysteresis can be observed at highly concentrated electrolyte solutions, due to the formation of a diffusion layer at the surface of the conducting electrode.
All the parameters from the BME680 as well as the plot obtained from the potentiostat could be displayed in the Wio Terminal. Additionally, the data extracted from the stripping voltammetry assay is displayed on the Sensor Station screen, alongside the slope of the obtained current pulse or the potential. The election of displaying such slope or peak can also be selected by the user. It must be noted tat, although only about 50 data points are displayed on the lcd screen, the potentiostat records up to 4000 points per experiments. Thus, the displayed results from the slope and peak may vary from the one expected observing the graphics on the screen. The code for this potentiostat is shown below.
4. Wireless powering of the potentiostat
One of the limitations of current potentiostats is the need of a power supply to carry out the experiments. One possibility to provide a constant power supply for portable applications is the use of batteries. However, they tend to have a short lifespan. Within this project, we proposed the use of inductive chargers to power the potentiostat proposed here. These inductive chargers consists of 2 coil inductors tat can be used to send or receive energy.
A promising application of these inductive chargers is the powering using a smartphone, since some recent smartphone devices already include an inductive charger module that can be used to provide energy to our potentiostat. However, in this case, we used another arduino Mega as to test the suitability of this system. In this case, only when both coils were placed next to each others, the potentiostat could be used.
After connecting both inductive chargers wirelessly, we could freely use our potentiostat. As such, we demonstrated the potential us of this system for portable applications.
5. Application of the sensor in smart gardening
A promising application of this low-cost potentiostat is the monitoring of electrolytes inside plant stems for precision irrigation applications. Due to the changes in the water content inside the plant stems, the electrical resistance can change. Such change can be measured using the low-cost potentiostat developed here. We calibrated the potentiostat using known resistances, and the inverse of the slope of the curve obtained after testing each resistance could be correlated (Figure below).
The calibration plot would allow the calculation of the resistance of the devices from the obtained slope of the potentiostat in different mediums. In this case, the screen-printed 3 electrode cell was inserted in the stem of a plant (Calathea). We compared these results with the ones obtained from a soil moisture sensor operating by inserting 2 metallic probes in the soil and measuring the input voltage after applying 5V.
In both cases, using the implanted electrode and the soil moisture sensor, a change in the obtained signal was observed after the water was added to the plant. In the case of the implanted electrode, and increase in the electrical resistance was observed, which is a consequence of the higher diffusion of water, that decreases the total concentration of dissolved ions inside the stem. A similar change in the output voltage can be observed in the case of the soil moisture sensor. However, the resistance in the stem decreased again after 1 h of having applied the water to the soil. On the contrary, the output voltage of the soil samples remained low, even after a few hours of watering the plant. As such, the measurement of the internal stem fluid could provide more information about the status of the plant, since the sudden decrease of the resistance of the stem could indicate a need of extra watering, which is not reflected on the soil measurements.”
