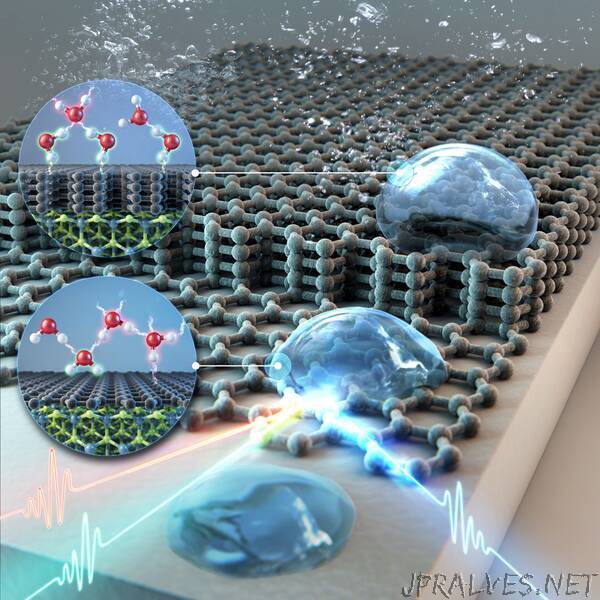
“Wettability of the material is the ability of a liquid to maintain contact with a solid surface, and it is proportional to hydrophilicity and inversely proportional to hydrophobicity. It is one of the most important properties of a solid, and understanding the wettability of different substrates is essential for various industrial uses, such as desalination, coating agents, and water electrolytes.
So far, studies on the wettability of substrates have mainly been measured at the macroscopic level. The macroscopic measurement of wettability is typically determined by measuring the water contact angle (WCA), which is the angle a water droplet makes with respect to the surface of the substrate. However, it is currently very difficult to accurately measure what happens at the interface between a substrate and water at the molecular level.
Currently used microscopic measurement techniques, such as reflection-based infrared spectroscopy or Raman spectroscopy, are incapable of selectively observing the interfacial water molecules. Since the number of water molecules in the entire bulk of the liquid is much larger than the molecules that are making contact with the surface, the signal of interfacial water molecules is obscured by the signal of water molecules in the bulk liquid.
To overcome this limitation, a research team at the Center for Molecular Spectroscopy and Dynamics (CMSD) within the Institute for Basic Science (IBS) in Seoul, South Korea, and the Korea University revealed that vibrational sum-frequency generation spectroscopy (VSFG) could be used for measuring the wettability of 2D-materials. The team succeeded in measuring the vibrational mode of water molecules in interfaces between graphene and water using VSFG spectroscopy.
VSFG is a useful technique that can connect the macroscopic measurement results with molecular-level properties. It is a surface-selective tool for investigating interfacial molecules using its own surface selection rule, and it has a very good surface resolution with a few molecular layers.
The group identified the unique ability of the graphene to project the wettability of the substrate onto its surface, which is called ‘wetting transparency’. They observed that the wetting transparency of graphene diminish as the number of graphene layers increased, disappearing when the graphene is more than 4 layers thick. This is the first observation to describe that graphene surface becomes hydrophobic above a certain number of layers at the molecular level.
Also, the researchers defined the new concept of VSFG wettability, which is the ratio of water molecules forming strong hydrogen bonds against water molecules with weak or no hydrogen bond formation. The VSFG wettability correlated strongly with the adhesion energy, which is calculated from the observed macroscopic WCA measurements. This proved that VSFG is an effective tool for defining the wettability of a material’s surface.
Using VSFG wettability, the researchers measured the wettability of the graphene in real-time, as an electric field was applied for it to form graphene oxide. It is impossible to observe wettability in real-time with the traditional WCA experiments. Therefore, this suggests that VSFG could be a decisive technique for measuring the water adhesion energy on any spatially confined interface where the water contact angle measurement cannot be applied. In addition to graphene, VSFG spectroscopy is expected to shed light on the wettability of other low-dimensional materials.
First author Eunchan Kim notes: “This study confirmed that VSFG spectroscopy could be used as a versatile tool for measuring the wettability.”, and “We demonstrate the potential to measure the wettability of previously unobservable complex systems through VSFG spectroscopy.”
Professor CHO Minhaeng, the Director of CMSD notes: “With VSFG spectroscopy, we are studying the microscopic properties of graphene as well as other two-dimensional functional materials such as graphene oxide and hexagonal boron nitride.”, and “Through this, it will be possible to solve various problems that hinder the commercialization of two-dimensional functional materials.”
This research was published in the online edition of Chem (IF 22.804) on April 26th.”
