“Lithium metal-based batteries have the potential to turn the battery industry upside down. With the theoretically ultra-high capacity of lithium metal used by itself, this new type of battery could power everything from personal devices to cars.
“In current batteries, lithium is usually atomically distributed in another material such as graphite or silicon in the anode,” explains Northwestern Engineering’s Jiaxing Huang. “But using an additional material ‘dilutes’ the battery’s performance. Lithium is already a metal, so why not use lithium by itself?”
The answer is a research challenge scientists have spent years trying to overcome. As lithium gets charged and discharged in a battery, it starts to grow dendrites and filaments, “which causes a number of problems,” Huang said. “At best, it leads to rapid degradation of the battery’s performance. At worst, it causes the battery to short or even catch fire.”
One current solution to bypass lithium’s destructive dendrites is to use a porous scaffold, such as those made from carbon materials, on which lithium preferentially deposits. Then when the battery is charging, lithium can deposit along the surface of the scaffold, avoiding dendrite growth. This, however, introduces a new problem. As lithium deposits onto and then dissolves from the porous support as the battery cycles, its volume fluctuates significantly. This volume fluctuation induces stress that could break the porous support.
Huang and his collaborators have solved this problem by taking a different approach — one that even makes batteries lighter weight and able to hold more lithium.
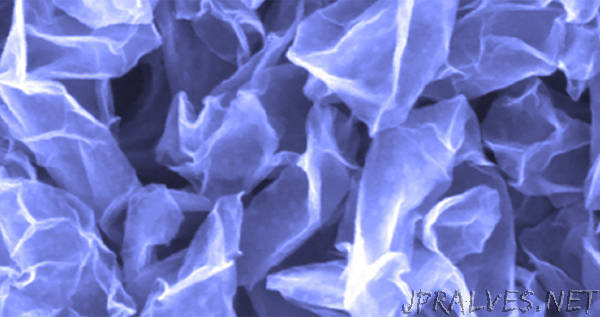
The solution lies in a scaffold made from crumpled graphene balls, which can stack with ease to form a porous scaffold, due to their paper ball-like shape. They not only prevent dendrite growth but can also survive the stress from the fluctuating volume of lithium. The research was featured on the cover of the January issue of the journal Joule.
“One general philosophy for making something that can maintain high stress is to make it so strong that it’s unbreakable,” said Huang, professor of materials science and engineering in Northwestern’s McCormick School of Engineering. “Our strategy is based on an opposite idea. Instead of trying to make it unbreakable, our scaffold is made of loosely stacked particles that can readily restack.”
Six years ago, Huang discovered crumpled graphene balls — novel ultrafine particles that resemble crumpled paper balls. He made the particles by atomizing a dispersion of graphene-based sheets into tiny water droplets. When the water droplets evaporated, they generated a capillary force that crumpled the sheets into miniaturized paper balls.
In Huang’s team’s battery, the crumpled graphene scaffold accommodates the fluctuation of lithium as it cycles between the anode and cathode. The crumpled balls can move apart when lithium deposits and then readily assemble back together when the lithium is depleted. Because miniature paper balls are conductive and allow lithium ions to flow rapidly along their surface, the scaffold creates a continuously conductive, dynamic, porous network for lithium.
“Closely packed, the crumpled graphene balls operate like a highly uniform, continuous solid,” said Jiayan Luo, the paper’s co-corresponding author and professor of chemical engineering at Tianjin University in China. “We also found that the crumpled graphene balls do not form clusters but instead are quite evenly distributed.”
Formerly advised by Huang, Luo earned his PhD in materials science and engineering in 2013. Now as a professor and researcher at Tianjin University, Luo continues to collaborate with Huang.
Compared to batteries that use graphite as the host material in the anode, Huang’s solution is much lighter weight and can stabilize a higher load of lithium during cycling. Whereas typical batteries encapsulate lithium that is just tens of microns thick, Huang’s battery holds lithium stacked 150 microns high.
Huang and his collaborators have filed a provisional patent through Northwestern’s Innovation and New Ventures Office (INVO).
The research was supported by the National Natural Science Foundation of China, the Natural Science Foundation of Tianjin, China, the State Key Laboratory of Chemical Engineering, and the Office of Naval Research.”
