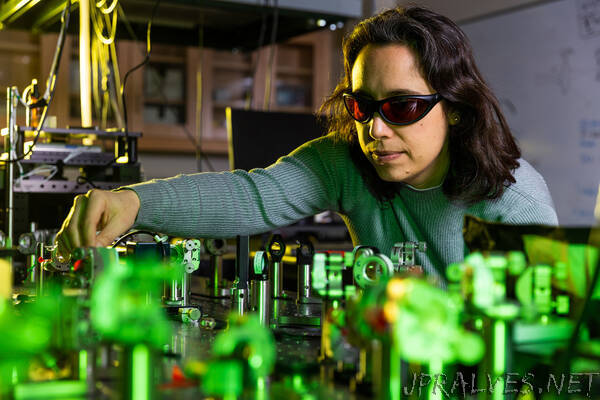
“Using ultrafast spectroscopy, the chemistry professor studies the energy transfer that occurs at femtosecond timescales inside plant leaves.
During photosynthesis, chlorophyll in plants absorbs packets of energy called photons from the sun’s rays. This energy is then transferred to a series of other chlorophyll molecules organized by protein scaffolds, funneling the energy into the next stage of photosynthesis.
Those early light-harvesting stages of photosynthesis involve repeated excitation of pigments, as photons are passed between them. To capture these highly dynamic processes, MIT Associate Professor Gabriela Schlau-Cohen employs ultrafast spectroscopy, a technique that uses extremely short laser pulses to study events that happen on timescales of femtoseconds to nanoseconds.
With this approach, Schlau-Cohen has made discoveries that reveal how photosynthesis is regulated under different light conditions, as well as how plants protect themselves from damage by dissipating excess sunlight.
“We are really interested in understanding the dynamics of electronically excited states, in photosynthesis and other systems,” she says. “We’re studying how energy can migrate through molecular systems and what controls the nature of that migration and its efficiency, particularly in the large protein networks that you find in photosynthesis.”
She also uses other spectroscopic techniques to study how proteins rapidly change their conformation when they bind to specific targets — for example, when receptors found on cell surfaces bind to stimuli such as growth factors or other signaling molecules.
Molecular interactions
As a high school student in the suburbs of Philadelphia, Schlau-Cohen enjoyed chemistry and was particularly intrigued by the phenomenon known as wave-particle duality: the concept that physical matter can have both wave-like and particle-like properties.
“I remember learning about wave-particle duality in my high school chemistry class, which is when I really became interested in chemistry. I had a really talented chemistry teacher who made all of the molecular interactions come alive,” she says.
At Brown University, she majored in chemical physics, which allowed her to explore the physical properties of molecules and molecular systems. There, she used ultrafast microscopy to study rapid processes such as energy moving between the electronic states of molecules.
After graduating from college, she spent three years in New York as a community organizer for the Working Families Party, where she worked on campaigns such as helping to raise the minimum wage for New York State.
“Social and economic justice causes were always something that was really important to me and that I was involved in throughout high school and college, so that was an interest that was present along with chemistry,” she says. “But as I was doing that work, I started to miss the intellectual challenge of science, and that led me to think about returning to science, so then I applied for grad school.”
She decided to go to the University of California at Berkeley, where she worked in a lab that used a type of ultrafast spectroscopy called multidimensional spectroscopy. Using this technique, she studied the energy transfer that occurs in photosynthetic light-harvesting complexes, down to the level of individual proteins within the complex.
“As we were studying these photosynthetic proteins, the simulations that I was doing in conjunction with the experimental work were showing that if you just looked at just one protein, the behavior of that protein was not just quantitatively but qualitatively different than what we could see in the ensemble,” she says.
As a postdoc at Stanford University, she went on to analyze the behavior of those individual photosynthetic proteins more closely, using single-molecule spectroscopy. She found that different copies of the same proteins could change shape, which changes how long they store energy from the sun.
Protein dynamics
When applying for faculty positions, Schlau-Cohen says she was drawn to MIT by the students’ talent and enthusiasm for science.
“When I visited MIT, one of the things that really stood out was the caliber of the students and the intellectual environment they were creating where we could have these really stimulating and exciting conversations about science,” she says. “Throughout MIT, there’s this real excitement about science and an interest in understanding how things work and how we can control how things work.”
Since starting her MIT lab in 2015, Schlau-Cohen has continued studying light-harvesting systems. She uses ultrafast spectroscopy to study how these systems transfer energy over long distances and how their efficiency is regulated in response to changes in sunlight. To help achieve that, she also works on improving the spectral bandwidth (which allows them to observe a wider range of energy levels) of ultrafast spectroscopy and the temporal resolution of single-molecule spectroscopy.
Her lab has published several papers in which they elucidated the mechanisms that allow plants to adjust the amount of energy captured from the sun when exposed to different weather conditions, and how they prevent sun damage. Single-molecule measurements of a protein called light-harvesting complex stress-related (LHCSR) revealed that it plays a key role in controlling these responses in green algae and moss.
Working with other MIT faculty members, including Mark Bathe, a professor of biological engineering, and Adam Willard, an associate professor of chemistry, she is also working on designing synthetic light-harvesting materials, using DNA origami structures as scaffolds.
“Our goal is to develop nanostructures with similar or even better emergent properties than photosynthetic light-harvesting systems, so that we can really achieve control over the evolution of light energy in a way that mimics or even exceeds the performance of nature,” she says.
In another area of research, Schlau-Cohen studies how proteins can respond to their environment by changing their structure. This shape shifting is a key element of cellular signal transduction systems, which control the flow of information within and between cells.
In one recent paper, she and Bin Zhang, an MIT associate professor of chemistry, analyzed how the epidermal growth factor receptor (EGFR) changes its conformation when it binds to its target. They discovered a large-scale structural shift that helps the receptor activate growth pathways inside the cell when activated by EGF.
“We’re interested in the structures of these proteins, and in how biological systems respond to changing environments by changing the structure and thus the function of their protein building blocks,” Schlau-Cohen says.”
