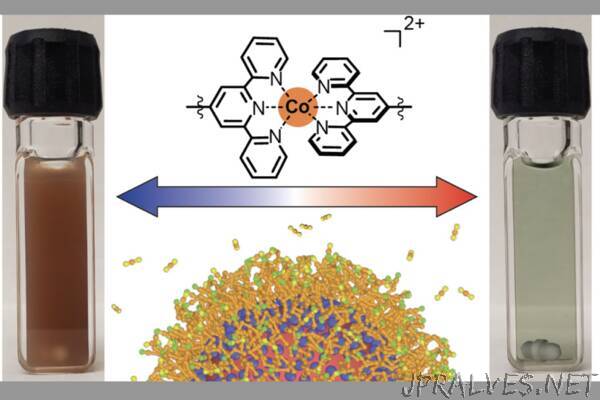
“New applications in energy, defense and telecommunications could receive a boost after a team at The University of Texas at Austin created a new type of “nanocrystal gel” — a gel composed of tiny nanocrystals each 10,000 times smaller than the width of a human hair that are linked together into an organized network.
The crux of the team’s discovery is that this new material is easily tunable. That is, it can be switched between two different states by changing the temperature. This means the material can work as an optical filter, absorbing different frequencies of light depending on whether it’s in a gelled state. So, it could be used, for example, on the outside of buildings to control heating or cooling dynamically. This type of optical filter also has applications for defense, particularly for thermal camouflage.
The gels can be customized for these wide-ranging applications because both the nanocrystals and the molecular linkers that connect them into networks are designer components. Nanocrystals can be chemically tuned to be useful for routing communications through fiber optic networks or keeping the temperature of spacecraft steady on remote planetary bodies. Linkers can be designed to cause gels to switch based on ambient temperature or detection of toxic substances in the environment.
“You could shift the apparent heat signature of an object by changing the infrared properties of its skin,” said Delia Milliron, professor and chair of the McKetta Department of Chemical Engineering in the Cockrell School of Engineering. “It could also be useful for telecommunications, which all use infrared wavelengths.”
The new research is published in the recent issue of the journal Science Advances.
The team, led by graduate students Jiho Kang and Stephanie Valenzuela, did this work through the university’s Center for Dynamics and Control of Materials, a National Science Foundation Materials Research Science and Engineering Center that brings together engineers and scientists from across campus to collaborate on materials science research.
The lab experiments allowed the team to see the material change back and forth between its two states of gel and not-gel (that is, free-floating nanocrystals suspended in liquid) that they triggered by specific temperature changes.
Supercomputer simulations done at UT’s Texas Advanced Computing Center helped them to understand what was happening in the gel at the microscopic level when heat was applied. Based on theories of chemistry and physics, the simulations revealed the types of chemical bonds that hold the nanocrystals together in a network, and how those bonds break when hit with heat, causing the gel to break down.
This is the second unique nanocrystal gel created by this team, and they continue to pursue advances in this arena. Kang is currently working to create a nanocrystal gel that can change between four states, making it even more versatile and useful. That gel would be a blend of two types of nanocrystals, each able to switch between states in response to chemical signals or temperature changes. Such tunable nanocrystal gels are called “programmable” materials.”
