“Solar perovskite materials could be spiked with organic cation dopants to suppress decomposition and degradation.
Expanding the list of organic ingredients that comprise perovskite solar materials could boost their long-term stability and performance, KAUST researchers have predicted.
Perovskites have recently captured the limelight in solar materials research because they can harvest solar energy almost as efficiently as conventional silicon solar cells and can be cheaper and easier to produce. One area where perovskites still lag behind silicon is their long-term stability. Now, Udo Schwingenschlögl from the KAUST Solar Center, and his Ph.D. student, Aleksandra Oranskaia, have found a possible solution.
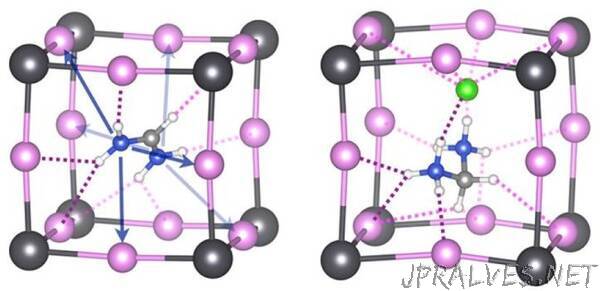
The most-studied perovskites for solar applications comprise a negatively charged lead-halide inorganic skeleton, partnered with positively charged organic cations, such as methylammonium (MA) or formamidinium (FA). These combine in a highly regular atomic arrangement. The lead halide component is mainly responsible for interacting with light, while the organic component plays a more supporting role, providing structural stability. However, the relatively poor stability of these materials still restricts their commercial development.
Using computational modeling, Oranskaia and Schwingenschlögl examined the organic component of solar perovskite materials, looking for ways to improve the stability of FA-lead halide perovskites. “Our motivation was to apply new computational methods to one of the hottest problems in the field of perovskite solar cells,” Oranskaia says.
Experimental studies have shown that FA-based perovskites are more stable than MA. Therefore, the team first compared MA and FA bonding strengths, focusing on noncovalent forms of bonding, such as hydrogen bonding. They then looked at whether adding other organic “dopants” into the FA-lead halide perovskite structure could enhance stability even further.
“We showed for the first time that the noncovalent bonding strength of organic cations can be used to improve hybrid perovskite materials,” Schwingenschlögl says. Although covalent bonds are the strongest, other types, including hydrogen bonding and halogen bonding between the organic cation dopants and the lead halide component, help to stabilize the perovskite structure.
“We show that doping with organic cations of the right volume and shape—those that bond more strongly than FA to the inorganic skeleton via hydrogen and halogen bonding—can stabilize the material,” Oranskaia says. Organic cations with covalently and noncovalently bound chlorine atoms or ions proved to be particularly effective: they helped to suppress damaging halide movements known as X-migrations. “This offers a strategy to boost the performance of lead halide solar cells,” Schwingenschlögl says.
The team next plans to study the effects of noncovalent interactions on the phase stability of other solar-related materials, Schwingenschlögl adds.”
