“Physicists at LMU Munich show that the shape of components is a major determinant of how quickly and efficiently complex structures self-assemble.
Complex systems in nature, like their synthetic counterparts in technology, comprise a large number of small components that assemble of their own accord through molecular interactions. Gaining a better understanding of the principles and mechanisms of this self-assembly is important for the development of new applications in domains such as nanotechnology and medicine.
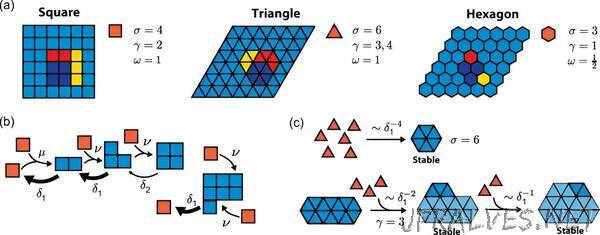
Professor Erwin Frey, Chair of Statistical and Biological Physics at LMU and member of the ORIGINS Excellence Cluster, and his research fellow Dr. Florian Gartner have now investigated an aspect of self-assembly that has received little attention before now: What role do the shape and the number of possible bonds between particles play? As the researchers report in the journal Physical Review X, their results show that hexagonal morphologies — in other words, six-sided structures — such as molecules with six binding sites are ideal for self-assembly.
Scaling phenomena pique interest of researchers
“When we investigated a general model of self-assembly, we observed that the assembly time increased with the size of the target structure,” recounts Gartner. “This made us wonder if the shape of the particles could have a considerable influence on how quickly the requisite assembly time increases with the size of the target structure and therefore how efficient the self-organization processes can be. This scaling of the assembly time with target structure size defines what we call the time complexity of self-assembly.”
Pursuing this thought, the scientists developed a mathematical model to analyze the behavior of the system during self assembly. Their results demonstrate that the morphology of the building blocks do in fact play an important role. By factoring in the scaling and kinetics of the systems, among other aspects, Frey and Gartner were able to show that hexagonal shapes offer considerable advantages for self-assembly. For example, the assembly of structures made up of a thousand building blocks can be almost four orders of magnitude faster with hexagonal building blocks compared to triangular ones.
This hexagon principle applies generally to the morphology, which not only describes the shape of the particles but also the number and positioning of their bonds: Six possible bonds to adjacent particles proved to be ideal when assembling larger structures. This can be covalent bonds, hydrogen bridge bonds, van der Waals forces, and hydrophobic interactions.
There are also correspondences in nature for this pattern, such as the self-assembly of viral capsids. This process starts with the assembly of small, triangular parts into hexagons, which subsequently join up with pentagons to form the icosahedral structures of viral capsids.
Potential applications
According to the scientists, their results furnish valuable insights for nanotechnology. The hexagon principle could be applied to optimize the self-organization of small structures into larger ones — as regards the shape of the building blocks or the possibility of bonds and adjacency relations with other particles. Through hierarchical self-assembly, for example, it could be possible to form particles with particularly advantageous morphology (hexagons for instance) in an initial assembly step in order to enhance the efficiency of the entire assembly process. “If you understand which morphologies of the monomers lead to efficient self-assembly, you can deliberately select these shapes and avoid inefficient shapes that are slow to assemble,” explains Gartner. “An example of how this strategy might be leveraged is in the synthesis of artificial viral capsids for biomedical applications.”“
