“In the realm of electric vehicles, powered by stored electric energy, the key lies in rechargeable batteries capable of enduring multiple charge cycles. Lithium-ion batteries have been the poster child for this application. However, due to limitations in energy storage capacity and other associated challenges, the focus has shifted to an intriguing alternative known as dual-ion batteries (DIBs).
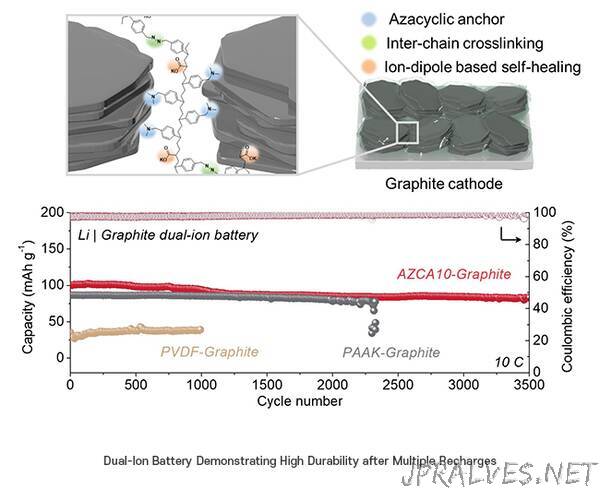
Dual-ion batteries utilize both lithium cations and counter anions simultaneously, offering a high energy density akin to traditional batteries. This allows them to store a substantial amount of energy. However, they face a hurdle due to the larger anions, causing expansion and contraction of the graphite anode material during charge and discharge, which can lead to decreased battery durability.
In a recent breakthrough, a collaborative research team tackled the durability issues of dual-ion batteries through innovative polymer binder research. The team included Professor Soojin Park (Department of Chemistry), alongside PhD candidates Jieun Kang (Department of Chemistry) and Jinwoo Hwang (Department of Chemical Engineering) from Pohang University of Science and Technology (POSTECH), Professor Jeong Woo Han (Materials Science and Engineering) from Seoul National University, and Professor Ja-Hyoung Ryu (Department of Chemistry) and Ph.D. student Seungho Lee (Department of Chemistry) from Ulsan National Institute of Science and Technology (UNIST), and Professor Jaegeon Ryu (Department of Chemical and Biomolecular Engineering) from Sogang University. The findings from this study were published in Advanced Materials.
The binder plays a critical role in securing various chemicals within rechargeable batteries. In this study, the research team introduced a novel polymer binder that incorporates azide groups (N3-) and acrylate groups (C3H3O2).
Azide groups form a robust covalent bond with graphite through a chemical reaction facilitated by ultraviolet light, ensuring the structural integrity of graphite during its expansion and contraction. Meanwhile, acrylate groups facilitate the reconnection between the graphite and the binder, even if the bond is disrupted.
Experimental results showed that dual-ion batteries equipped with the newly developed binder maintained exceptional performance, even after enduring over 3,500 recharge cycles. These batteries also demonstrated swift charging capabilities, with about 88% of the original capacity being restored within just 2 minutes.
Professor Soojin Park, the driving force behind the research, explained, “Dual-ion batteries are not only cost-effective but also leverage Earth’s abundant graphite resources. This research will stimulate further exploration of dual-ion batteries, extending beyond electric vehicles to various other applications.”
Importantly, this research was made possible with support from the Mid-Career Researcher Program and the C1 Gas Refinery Program of the National Research Foundation of Korea (NRF) under the Ministry of Science and ICT, as well as the Basic Science Research Program (Doctoral Program Research Grant) of the NRF under the Ministry of Education of Korea.”
