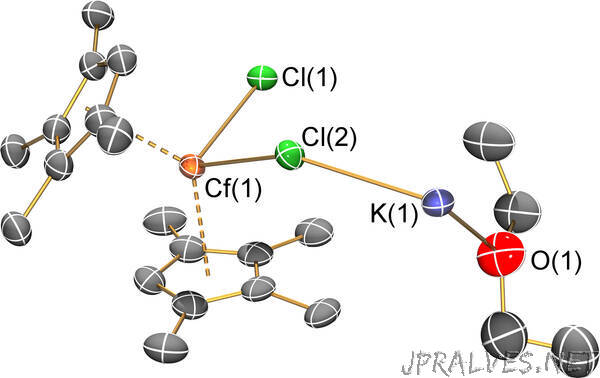
“Challenging study revealed structure, bonding trends and reactivity
New work led by Los Alamos National Laboratory, in the journal Nature this week, reports the first structural characterization of californium-carbon bonding in a molecule. Such analysis allows scientists to assess periodic reactivity and bonding trends in an otherwise mysterious element.
“Californium captures the imagination and offers broad science appeal because of its exotic place in the periodic table,” said Andrew Gaunt, a Los Alamos chemist and one of the paper’s lead authors. “Due to the scarcity of well-characterized molecules, we are testing and expanding the known limits of chemical bonding and electronic structure for an element that has largely eluded definition.”
He added, “Organometallic molecules have proven foundational in understanding the chemical properties of many metals. Conducting organometallic studies to californium allows an unprecedented comparison to earlier, more accessible actinide chemistry such as with thorium and uranium, and heavy lanthanide analogues. Via these side-to-side comparisons we can begin to assess periodic reactivity and bonding trends that have sat frustratingly out of reach for decades.”
Californium is the heaviest element that, although very scarce, is available on a milligram scale, just large enough to conduct classical synthetic chemistry – that is, to isolate molecules in pure single-crystal form, determine their structures (how atoms in a molecule are arranged in space and connected to each other), and acquire spectroscopic data that, coupled to quantum mechanical calculations, informs about the distribution and properties of electrons in the molecules.
The synthesis was performed with 2-milligrams of californium, a quantity that is typically considered tiny for organometallic chemistry involving more abundant elements. This feat was possible as a result of a capability built over years of program development to conduct modern-era transuranium organometallic chemistry at Los Alamos. The small scale is driven by the scarce availability of californium isotopes and the radiation hazards they pose. “Such rare experimental data on californium provided a foundation for computational modeling to assess subtle bonding and electronic structure differences in californium molecules compared to similarly sized heavy lanthanides and also lighter actinides,” Gaunt said.
Considering the substantial radiation hazards of the Cf-249 isotope studied here, experimentation is logistically very challenging and had to be highly choreographed. Given that very few californium molecules have ever been structurally characterized, the organocalifornium (californium bonded to carbon atoms) compound reported here allows a rare assessment of reactivity and bonding trends deep into the actinide series and represents a frontier of isolable molecules in the periodic table.
Paper: Isolation and characterization of a californium metallocene, Nature, Nov. 17, 2021 online, print date Nov. 18, 2021.
Funding: Work funded at Los Alamos National Laboratory by the U.S. Department of Energy (DOE), Office of Science (SC), Basic Energy Sciences (BES) Heavy Element Chemistry Program (HEC) at LANL. Conrad Goodwin was sponsored by a Distinguished J. R. Oppenheimer Postdoctoral fund. Aspects of the associated Am-241 experimental work were funded LANL LDRD. Theoretical research was performed using the Environmental Molecular Science Laboratory (EMSL), a DOE-SC User Facility sponsored by the Office of Biological and Environmental Research. Am-241 sample conditioning and dispensing was funded by DOE-SC, Isotope Development and Production for Research and Application subprogram within the Office of Nuclear Physics, and the NNSA Plutonium Sustainment Program and the National Nuclear Security Administration (NNSA) Material Recycle and Recovery.”
