“As science advances, ever more accurate measurements are both needed and achievable. But this improving accuracy must happen through measurement standards and their definitions.
In November 2018, at the General Conference on Weights and Measures, the global metrology community agreed a revision to the SI.
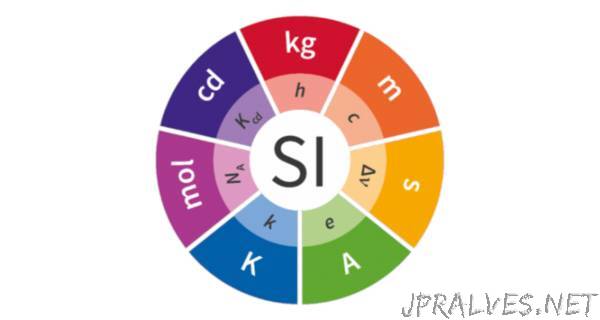
The decision means that, for the first time, all seven of the base units will be defined in terms of constants of nature – such as the speed of light, the Planck constant and the Avogadro constant. Using seven defining constants as the basis for the SI will mean that the definitions of all the base units will stay stable into the future.
The revision will bring in new definitions of the ampere, kilogram, kelvin (and, consequently, degree Celsius) and mole.
Although these changes won’t be felt in everyday life, they represent a profound change of perspective. From May 2019, all the base units of the SI will be defined in terms of constants of nature – the most stable quantities we have ever encountered.
Redefinition
Making this revision across the whole SI is a profound change in approach that will underlie all measurements in science and more widely. These changes will be used by scientists and engineers who are making measurements at the extremes, but in everyday life it will appear that not much has changed. The changes in the SI will ensure that the SI definitions stay robust for the future, ready for advancements in science and technology. The new definitions impact four of the base units:
The kilogram – will be defined in terms of the Planck constant (h)
The ampere – will be defined in terms of the elementary charge (e)
The kelvin – will be defined in terms of the Boltzmann constant (k)
The mole – will be defined in terms of the Avogadro constant (NA)”
Link to article
