“Uppsala researcher Dr Laurent Duda and PhD student Felix Massel together with researchers at the University of Oxford, the University of Kent and the Paul Scherrer Institute have come a long way in finding future lithium-free batteries for devices such as smartphones and laptops.
Smartphone and laptop batteries operate by light alkali ions shuttling back and forth between the anode and cathode. For this purpose, the rechargeable batteries of today use lithium, which is the lightest and smallest of all metal ions. The abundance of lithium salts on Earth is probably insufficient for satisfying the future total demand of energy storage in the world. Therefore it is crucial to study and increase the capacity of battery cathodes based on other light ions. Such ions are for example magnesium and also sodium, an alkali ion provided plentifully by the world’s oceans and in table salt.
An early breakthrough
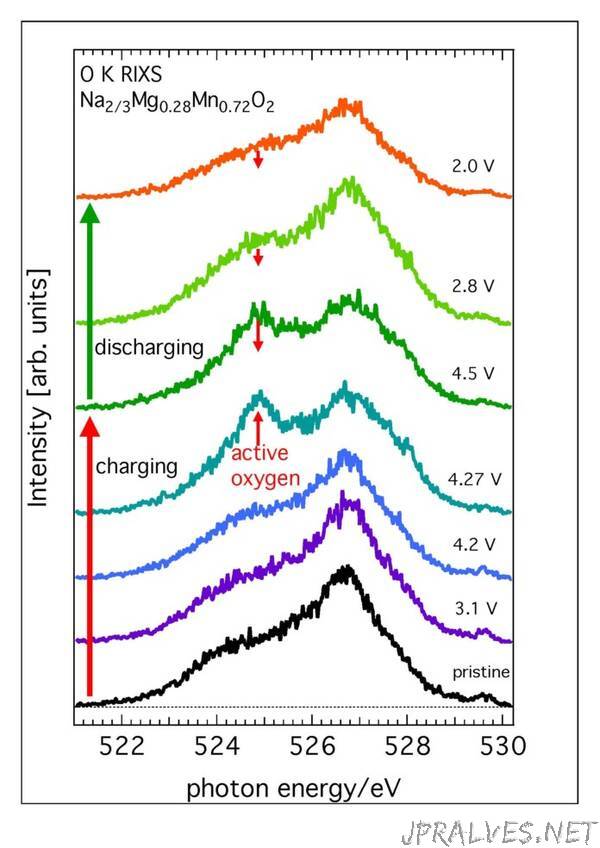
Modern rechargeable batteries made of metal oxides are limited in their capacity since only the metal ions of the cathode are active. An early breakthrough regarding increased battery capacity was achieved little less than two years ago. At that time part of the research team involved in the new study could show that oxygen ions in lithium rich battery cathodes, lend these cathodes their so called extra capacity. However, lithium rich cathodes lose a part of their extra capacity in repeated charge cycles. This is due to the fact that part of the lithium participating in the battery cycling resides closely to the oxygen. When the battery is charged and these lithium ions are removed from the cathode, the oxygen becomes instable and the cathode gradually degrades.
“Many researchers thought that this degrading of the cathode was inevitable and related to the extra capacity of the battery,” says Laurent Duda, researcher at the Department of Physics and Astronomy.
A stable, lithium-free material
The new study, which looks at the lithium-free material Na2/3[Mg0.28Mn0.72]O2 points to the opposite though. It shows that lithium, or any other kind of alkali ion, does not have to be stored close to the oxygen in a battery that has extra capacity. The studied material is completely free from lithium and instead has sodium ions going back and forth. At the same time the magnesium ions close to the oxygen have a stabilising influence on the material.
The researchers at Oxford have synthesised the studied lithium-free material and together with methods of characterisation used by the Kent researchers they could confirm that the material had the desired extra capacity and was extremely stable too.
Further optimisation needed
To confirm that the oxygen indeed was the cause of the extra battery capacity, and to rule out other explanations, the Uppsala researchers used a technique known as resonant inelastic X-ray scattering or RIXS. The Uppsala researchers Laurent Duda and Felix Massel at the Department of Physics and Astronomy have contributed a lot to the strong development of this technique during the last decades. The RIXS-experiment was carried out with world leading equipment on the beamline ADRESS at the Swiss synchrotron radiation laboratory Swiss Light Source.
“Discovering that extra capacity also may be found in lithium-free batteries is a huge progress, but further optimisation of lithium-free cathode materials is needed before they become viable for commercial batteries. Further RIXS-experiments will contribute a lot to our work on this in the close future,” says Laurent Duda.”
