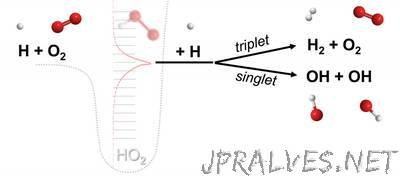
“Finding opens door to innumerable possibilities, from the design of new types of engines to understanding the planetary chemistry responsible for cloud formations, climate change, and the conditions for creating life. A new study led by Michael P. Burke, assistant professor of mechanical engineering at Columbia Engineering, has identified the significance of a new class of chemical reactions involving three molecules that each participate in the breaking and forming of chemical bonds. The reaction of three different molecules is enabled by an “ephemeral collision complex,” formed from the collision of two molecules, which lives long enough to collide with a third molecule. This fourth class, which the researchers have named “chemically termolecular reactions,” was first hypothesized by Cyril Hinshelwood and Nikolay Semenov in their studies of chain reactions in the 1920s and 30s (they won the 1956 Nobel Prize in Chemistry for this work). For decades, researchers have considered these reactions unimportant—if they even occurred at all—and until now, no one has studied them. Burke, who explores a variety of problems at the interface between fundamental physical chemistry and practical engineering devices, decided to investigate these reactions after realizing that common combustion situations, such as those encountered in many engines, have sufficiently high fractions of highly reactive molecules known as free radicals to make these reactions possible. The new study is published today in Nature Chemistry. “Combustion has always been a launching point for understanding all sorts of other chemical mechanisms,” says Burke, who is also a member of the Data Science Institute. “Potentially there could be innumerable reactions from this new class that impact how we model gas phase chemistry, from designing new types of engines to understanding the planetary chemistry responsible for cloud formations, climate change, evolution of pollutants, even perhaps the sequence of reactions that could impact the conditions for extraterrestrial life. Our discovery opens up a whole new world of possibilities.” For example, space vehicles experience very high temperatures and radical fractions in their descent back to Earth. Burke speculates that this fourth class of reactions could impact the heat flux to the vehicle, with significant implications for the design of thermal protection systems to keep astronauts and/or payloads safe when coming down to Earth. Working with Stephen J. Klippenstein, (Chemical Sciences and Engineering Division, Argonne National Laboratory), Burke used state-of-the-art computational methods, combining quantum chemical computations that simulate the breaking and forming of chemical bonds among reacting molecules with kinetic-transport computations that simulate the reactions and movements of bulk gases that governs performance of engineering devices.”
