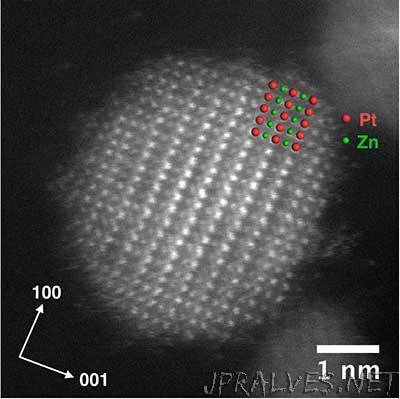
“Scientists at Ames Laboratory have discovered a method for making smaller, more efficient intermetallic nanoparticles for fuel cell applications, and which also use less of the expensive precious metal platinum. The researchers succeeded by overcoming some of the technical challenges presented in the fabrication of the platinum-zinc nanoparticles with an ordered lattice structure, which function best at the small sizes in which the chemically reactive surface area is highest in proportion to the particle volume. “That surface-to-volume ratio is important in getting the most out of an intermetallic nanoparticle,” said Wenyu Huang, Ames Laboratory scientist and assistant professor of Chemistry at Iowa State University. “The smaller the particle, the more surface there is, and more surface area increases the catalytic activity.” But the high temperature of the annealing process necessary to form intermetallic nanoparticles often defeats the goal of achieving a small size.”
