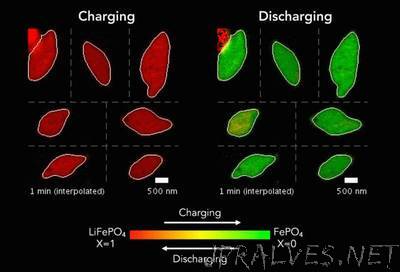
“Better batteries that charge quickly and last a long time are a brass ring for engineers. But despite decades of research and innovation, a fundamental understanding of exactly how batteries work at the smallest of scales has remained elusive. In a paper published this week in the journal Science, a team led by William Chueh, an assistant professor of materials science and engineering at Stanford and a faculty scientist at the Department of Energy’s SLAC National Accelerator Laboratory, has devised a way to peer as never before into the electrochemical reaction that fuels the most common rechargeable cell in use today: the lithium-ion battery.”
