“Frozen water can take on up to three forms at the same time when it melts: liquid, ice and gas. This principle, which states that many substances can occur in up to three phases simultaneously, was explained 150 years ago by the Gibbs phase rule. Today, researchers from Eindhoven University of Technology and University Paris-Saclay are defying this classical theory, with proof of a five-phase equilibrium, something that many scholars considered impossible. This new knowledge yields useful insights for industries that work with complex mixtures, such as in the production of mayonnaise, paint or LCD’s. The researchers have published their results in the journal Physical Review Letters.
The founder of contemporary thermodynamics and physical chemistry is the American physicist Josiah Willard Gibbs. In the 1870s he derived the phase rule, which describes the maximum number of different phases a substance or mixture of substances can assume simultaneously. For pure substances, the Gibbs Phase Rule predicts a maximum of 3 phases.
Professor Remco Tuinier, of the Institute for Complex Molecular Systems: “At the time, Einstein called Gibbs’ thermodynamics the only theory he really trusted. If we take water as an example, there is one point, with a specific temperature and pressure, where water occurs as gas, liquid and ice at the same time. The so-called triple point.” Assistant professor Mark Vis, from the same research group as Tuinier, adds: “This classic Gibbs phase rule is as solid as a rock and has never been defied.”
SHAPE MATTERS
According to this phase rule, the mixture studied by the researchers would also exhibit a maximum of three phases at one specific point at the same time. But Tuinier and his colleagues now show that in this mixture there is a whole series of circumstances in which four phases exist at the same time. There is even one point at which there are five coexisting phases. Two too many, according to Gibbs. At that specific one point, also called a five-phase equilibrium, a gas phase, two liquid crystal phases, and two solid phases with ‘ordinary’ crystals exist simultaneously. And that has never been seen before. “This is the first time that the famous Gibbs rule has been broken,” Vis says enthusiastically.
The crux lies in the shape of the particles in the mixture. Gibbs did not take this into consideration, but the Eindhoven scientists now show that it is precisely the specific length and diameter of the particles that play a major role. Tuinier: “In addition to the known variables of temperature and pressure, you get two additional variables: the length of the particle in relation to its diameter, and the diameter of the particle in relation to the diameter of other particles in the solution.”
RANKED RODS
In their theoretical models, the researchers worked with a mixture of two substances in a background solvent: rods and polymers. This is also called a colloidal system, in which the particles are solid and the medium is liquid. Because the particles cannot occupy exactly the same space, they interact with each other. “This is also called the excluded volume effect; it causes the rods to want to sit together. They are, as it were, pushed towards each other by the polymer chains. In this way, you get a region in the mixture that mainly contains rods, and an area that is rich in polymers,” explains Tuinier.
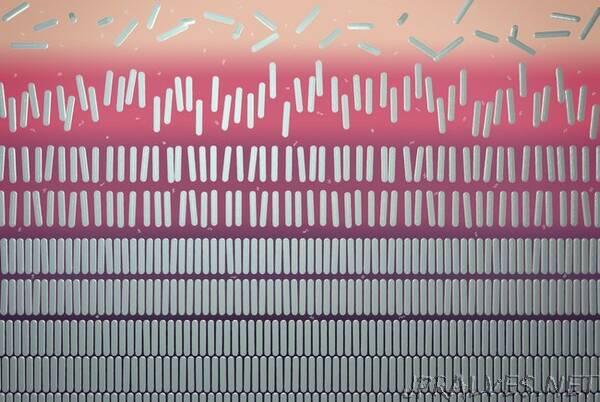
He continues: “The rods then sink to the bottom, because they’re usually heavier. That’s the beginning of segregation, creating phases.” The lower part, which mainly contains rods, will eventually become so crowded that the rods will interfere with each other. They then take up a preferential position, so that they are less in each other’s way.
With the rods it looks like a neat arrangement next to each other. Eventually you get five different phases, a gas phase with unaligned rods at the top (an isotropic phase), a liquid phase with rods pointing in about the same direction (nematic liquid crystal), a liquid phase with rods lying in different layers (smectic liquid crystal), and two solid phases at the bottom.
MAYONNAISE AND MONITORS
Vis: “Our research contributes to the fundamental knowledge about this kind of phase transition and helps to understand and predict more precisely when these kinds of transition occur.” And that is useful in many areas. Think of pumping complex mixtures around in industrial reactors, making complex products like colloidal mixtures such as mayonnaise and paint, or ice that forms on car windows and black ice on roads.
Even in liquid crystals in monitors, these processes play a role. “Most industries choose to work with a single-phase system, where there is no segregation. But if the exact transitions are clearly described, then the industry can actually use those different phases instead of avoiding them,” says Vis.
CHANCE
It was more or less chance that the researchers arrived at an equilibrium of more than three phases. When simulating and programming plate-shaped particles and polymers, PhD students Álvaro González García and Vincent Peters from Tuinier’s group saw a four-phase equilibrium. Tuinier: “Álvaro came to me one day and asked me what had gone wrong. Because four phases just couldn’t be right.”
Then the researchers tried out multiple shapes, such as cubes and also rods. Tuinier: “With the rods, most phases turned out to be possible, we even found a five-phase equilibrium. That could also mean that even more complicated equilibria are possible, as long as you search long enough for complex different particle shapes.”
This research appeared on 18 September in the journal Physical Review Letters under the title ‘Defying the Gibbs Phase Rule: Evidence for an Entropy-Driven Quintuple Point in Colloid-Polymer Mixtures’.
DOI: 10.1103/PhysRevLett.125.127803
The research has been carried out at Eindhoven University of Technology, at the Department of Chemical Engineering and Chemistry and the Institute for Complex Molecular Systems, and at Paris-Saclay University.”
