“With user facilities, researchers devise novel battery chemistries to help make fluoride batteries a reality.
The Science
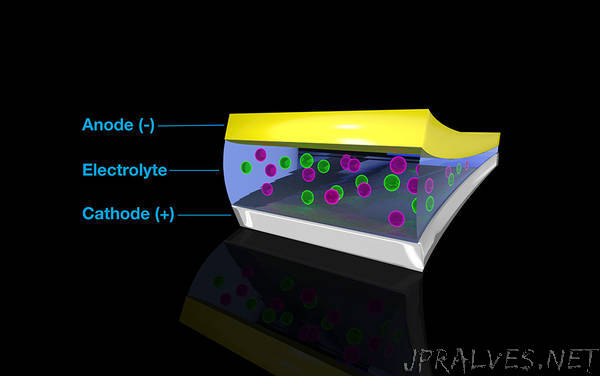
Energy-dense fluoride batteries are exciting, but they only work at high temperatures. A collaboration of researchers recently discovered a new liquid electrolyte. It conducts fluoride in fluoride-based rechargeable batteries at room temperature. These batteries pack a major energy punch. As part of the study, the team used three Department of Energy user facilities. For example, they used the Titan supercomputer at the Oak Ridge Leadership Computing Facility to understand and refine the electrolyte’s unprecedented ability to conduct fluoride ions and retain chemical stability at room temperature. The breakthrough material is the first of its kind in the battery world.
The Impact
Energy-dense fluoride batteries could hold up to eight times more charge than lithium batteries. Simulations such as the ones done can help scientists understand battery interactions and improve existing and newly discovered electrolytes. The understanding of the new electrolyte, called BTFE, gives researchers a map for the mechanisms involved in stabilizing fluoride batteries. Also, it could aid in the development of new kinds of batteries for use in cars, cell phones, or other electronic devices.
Summary
Researchers discovered a new liquid electrolyte named BTFE. The electrolyte could replace the molten salt currently used in fluoride-based batteries. The researchers studied the electrolyte and related materials using scanning transmission electron microscopy/electron energy loss spectroscopy at the Molecular Foundry. They used the computing resources at National Energy Research Scientific Computing Center and the Oak Ridge Leadership Computing Facility to run complex calculations and simulations. For example, they conducted molecular dynamics simulations on the Titan supercomputer to look at BTFE’s reactions with fluoride. The new solvent worked and made the fluoride ions stable enough to shuttle from the battery’s anode to the cathode, but the team didn’t quite know why before the simulations. They found that positively charged regions in BTFE were strongly interacting with the negatively charged fluoride to dissolve it. The team simulated many different solvents and positively charged ions—including a solvent that could potentially expand the voltage window of BTFE and make it even more stable. Follow-up studies on the project can aid in the optimization of new liquid electrolytes such as BTFE, enabling the possibility of fluoride-based batteries.”
