“Odorless route to polymeric delivery systems is safer and cheaper
An affordable, heavy metal- and odor-free method for making hollow polymer nanostructures has been designed by A*STAR researchers. These structures could find use as delivery systems for personal care products, drugs and agrochemicals.
The use of hollow polymer nanostructures for carrying — and preserving the stability of — active ingredients, such as salicylic acid, vitamins, drugs and pesticides, is booming. These shells release cargo on demand in response to triggers such as water. Current manufacturing methods are imperfect, due to either cost, environmental or safety reasons. Significant efforts are therefore being devoted to developing alternatives.
Alexander van Herk from the A*STAR Institute of Chemical and Engineering Sciences and Atsushi Goto from Nanyang Technological University, have designed a safe, simple route to synthesize polymer nanoparticles, nanocylinders and nanocapsules.1
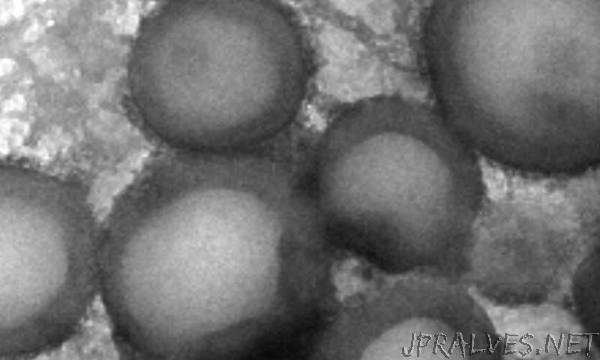
The first stage in this two-step synthesis is to polymerize methylacrylic acid (MAA) in the presence of iodine to make alkyl iodides. The poly(methacrylic acid) (PMAA) then acts as an initiator in the polymerization of methyl methacrylate (MMA) and formation of the block co-polymer. Sodium iodide is the catalyst in both stages. PMAA is hydrophilic and poly(methyl methacrylate) (PMMA) is hydrophobic, meaning that in polar solvents such as ethanol and water the co-polymer self-assembles into hollow nanostructures.
By varying the polymer proportions, van Herk and Goto could determine the nanostructures’ shape. The lowest portion of PMMA to PMAA gave nanoparticles; boosting the hydrophobic content led to nanocylinders, then nanocapsules. The team also found that tweaking the two polymer lengths enabled control over structure size and shell thickness.
The use of iodine is the most innovative part of this work. Sodium iodide is highly active, biologically safe, inexpensive, colorless and odorless. Current polymerizations either use smelly sulfur containing groups, or heavy metal ions such as copper. “Utilizing iodine mediated controlled radical polymerization gives us sulfur and metal-free capsules which have broader acceptance than those produced with the previous methods,” says van Herk.
The team repeated the experiments adding a small amount of cross-linkable hydrophobic monomer ethylene glycol dimethacrylate to the MMA in the second polymerization step. “Crosslinking is important to ‘freeze’ the structure, to ensure it doesn’t change during further handling,” van Herk says. This led to more stable nanostructures.
The delivery capabilities of these polymers have not yet been tested. “So far we have not loaded these capsules with active compounds such as vitamins or peptides. The next step could be to do so, and then study the release profiles,” says van Herk.”
