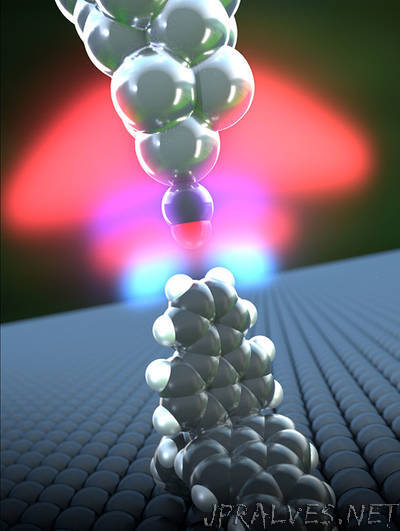
“For the first time, scientists have succeeded in studying the strength of hydrogen bonds in a single molecule using an atomic force microscope. Researchers from the University of Basel’s Swiss Nanoscience Institute network have reported the results in the journal Science Advances. Hydrogen is the most common element in the universe and is an integral part of almost all organic compounds. Molecules and sections of macromolecules are connected to one another via hydrogen atoms, an interaction known as hydrogen bonding. These interactions play an important role in nature, because they are responsible for specific properties of proteins or nucleic acids and, for example, also ensure that water has a high boiling temperature. To date, it has not been possible to conduct a spectroscopic or electron microscopic analysis of hydrogen and the hydrogen bonds in single molecules, and investigations using atomic force microscopy have also not yielded any clear results. Dr. Shigeki Kawai, from Professor Ernst Meyer’s team at the Swiss Nanoscience Institute and the Department of Physics at the University of Basel, has now succeeded in using a high-resolution atomic force microscope to study hydrogen atoms in individual cyclic hydrocarbon compounds.”
Link to article
