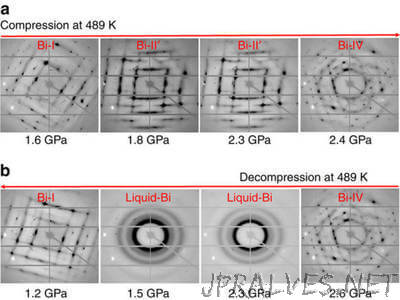
“Phase transitions surround us—for instance, liquid water changes to ice when frozen and to steam when boiled. Now, researchers at the Carnegie Institution for Science* have discovered a new phenomenon of so-called metastability in a liquid phase. A metastable liquid is not quite stable. This state is common in supercooled liquids, which are liquids that cool below the freezing point without turning into a solid or a crystal. Now, scientists report the first experimental evidence of creating a metastable liquid directly by the opposite approach: melting a high-pressure solid crystal of the metal bismuth via a decompression process below its melting point. The results, reported in the January 23, 2017, issue of Nature Communications, could be important for developing new materials and for understanding the dynamics of planetary interiors, such as earthquakes, because a metastable liquid could act as a lubricant strongly affecting the dynamics of the Earth’s interior.”
